Monitoring of Polymerization reactions
From LEAP
(→Overview) |
|||
| Line 9: | Line 9: | ||
=== Overview === | === Overview === | ||
| - | Reaction monitoring of polymers, chemicals, and fermentation broths is a vital part of product development. Often times these reactions are at elevated temperatures and pressures in the presence of catalysts. Reactions can take a couple hours up to a full day to complete. Samples often need quenching to stop the reaction at specific time points. This application is geared toward a cost effective yet reliable way of taking timed samples and quenching each sample with optional just-in-time chemical analysis by either gas or liquid chromatography. | + | Reaction monitoring of polymers, chemicals, and fermentation broths is a vital part of product development. Often times these reactions are at elevated temperatures and pressures in the presence of catalysts. Reactions can take a couple hours up to a full day to complete. |
| + | Quenching: Samples often need quenching to stop the reaction at specific time points. This application is geared toward a cost effective yet reliable way of taking timed samples and quenching each sample with optional just-in-time chemical analysis by either gas or liquid chromatography. | ||
One such reaction is the catalytic conversion of a mixture of monomers to a mixture of polymers of specific chain lengths to achieve the properties needed for a specific application. Typical reaction conditions include temperatures of 100 C, pressures of 100 psi, and 18 hour reaction durations. Often very volatile and explosive solvents are involved which presents a very real safety issue when unattended timed samples are taken. It is also important that the sampling does not interfere with the polymeric reaction. | One such reaction is the catalytic conversion of a mixture of monomers to a mixture of polymers of specific chain lengths to achieve the properties needed for a specific application. Typical reaction conditions include temperatures of 100 C, pressures of 100 psi, and 18 hour reaction durations. Often very volatile and explosive solvents are involved which presents a very real safety issue when unattended timed samples are taken. It is also important that the sampling does not interfere with the polymeric reaction. | ||
Revision as of 14:16, 7 May 2009
| Monitoring of Polymerization reactions |
| Application Type | |
| SPECIAL | |
| Application ID | |
| Description | |
| Timed sampling of polymer reactor vessel under high pressure and temperature. |
Contents |
Overview
Reaction monitoring of polymers, chemicals, and fermentation broths is a vital part of product development. Often times these reactions are at elevated temperatures and pressures in the presence of catalysts. Reactions can take a couple hours up to a full day to complete. Quenching: Samples often need quenching to stop the reaction at specific time points. This application is geared toward a cost effective yet reliable way of taking timed samples and quenching each sample with optional just-in-time chemical analysis by either gas or liquid chromatography. One such reaction is the catalytic conversion of a mixture of monomers to a mixture of polymers of specific chain lengths to achieve the properties needed for a specific application. Typical reaction conditions include temperatures of 100 C, pressures of 100 psi, and 18 hour reaction durations. Often very volatile and explosive solvents are involved which presents a very real safety issue when unattended timed samples are taken. It is also important that the sampling does not interfere with the polymeric reaction.
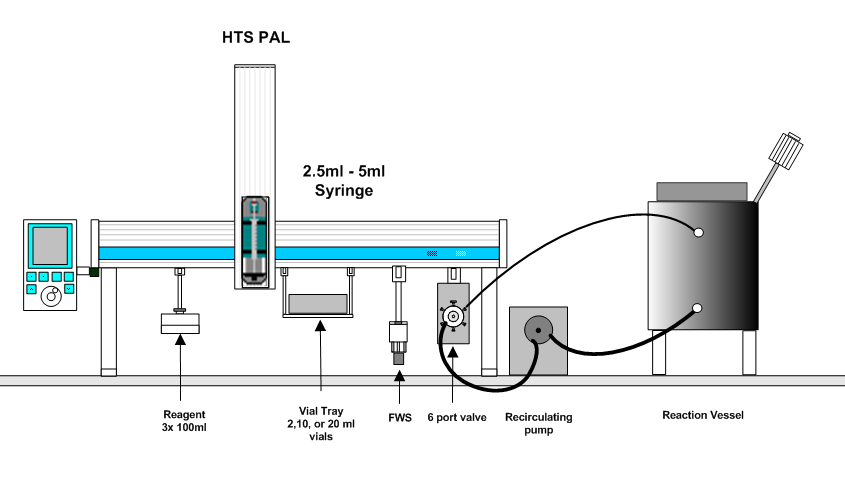
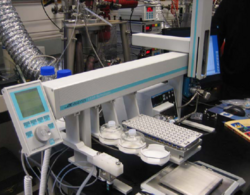
LEAP has broken new ground in one such application, where the complexities of this type of reaction monitoring has been fully automated using a PAL workstation equipped with a sampling valve, a gear pump, and timing software to control the whole process.
Market/Applications
- Method development in many industries,
- Pharma, Bio Tech, Polymer.
- QC at production sites.
- Process stream monitoring in plant or waste water discharge stream.
The Process
The reaction monitoring process begins with the operator creating a sample list with the desired number of timed samples and the desired amount of reactant for each time point. The timing software automatically calculates the amount of quench reagent to add and the resulting dilution factor of the reactant. The Pal then pre-fills the proper number of 2ml chromatography glass vials with the appropriate amount of quenching solvent. A time zero sample point is taken and the software prompts the operator to add the catalyst. The Pal then proceeds to take the proper number of samples at the proper times and deposits each sample into a 2ml vial with the quenching reagent. The sampling system is cleaned thoroughly between each sample to insure that none of the quenching solvent enters into the reactor. The recirculating flow rate is such that each timed sample is representative of the contents of the reactor. LEAP Shell handles the sample scheduling of each of the three stages of the sampling process, time stamps and logs each step to a permanent file, and prepares an export file suitable to import into the chromatographic data acquisition software. The whole process is automated in a safe and reliable manner so that an operator does not have to work late into the night.
The whole system can be placed upon a movable cart and positioned close to any reactor.
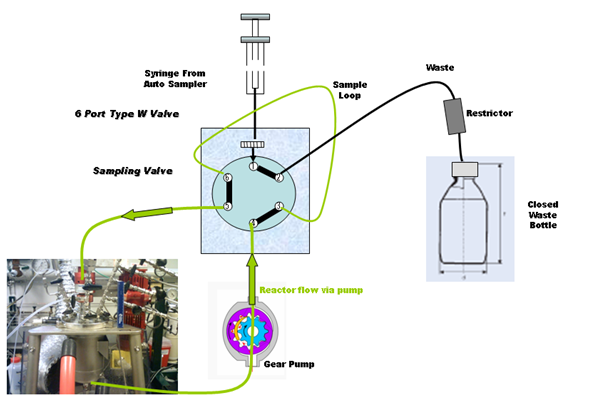
Flow Diagram in default position (Loop being flushed):
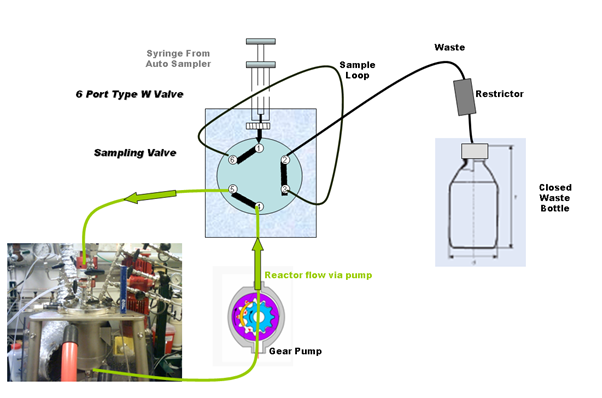
Flow diagram when sampling from loop:
The waste container with restrictor is to trap expansion liquid/gas in the case where the reaction mixture is under pressure.
Overall System Layout:
Screen Shots
Further Documentation
Contact LEAP
For additional information about this technique please contact LEAP Technologies for detailed information